Value Demonstration
Our clients trust us to plan, develop and communicate evidence and we are proud of our role in gaining patients access to innovative new treatments. We bring strategic thinking, deep technical expertise and creative flair, turning insights into actions and developing outputs that resonate with payers.
Service offerings
Planning

Access strategy
We work with payers, policy-makers, clinicians, health economists and patients to understand their thoughts and beliefs around the planned indication, the drivers of their decisions and the information they will need to achieve access for patients. Working closely with internal teams, we combine learnings to develop market access strategy and evidence planning.
Integrated evidence generation planning
We are leaders in developing integrated evidence generation plans. We partner with our clients from strategic planning, through evidence planning workshops to delivery of integrated plans that include the evidence needs and activities that are critical for market access.

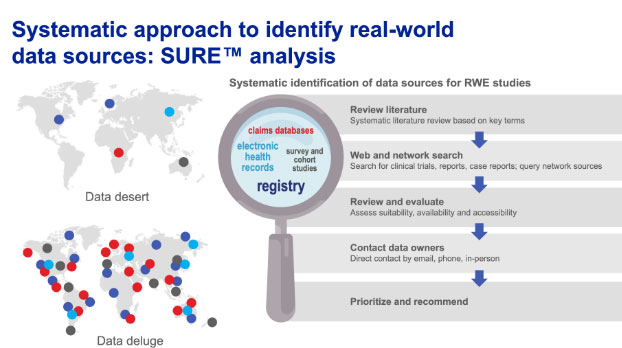
SURETM real-world data source identification
We have specialist expertise in identifying and prioritizing real-world data sets and providing unbiased recommendations for their use in evidence generation activities.
SURETM real-world data source identification
Our SURE™ assessments follow a systematic approach to identify real-world data sources and rank by relevance and utility based on objective criteria.
Our robust methodology allows transparent justification of the selection of real-world data sources in line with real-world evidence frameworks.



Payer landscape assessments
We have expertise in upskilling internal teams with the information they need when developing market access strategies. We gather evidence from published and unpublished sources, clearly structuring and communicating the findings to inform development decisions.
Payer landscape assessment
We gather and generate insights assisted by carefully selected AI and other technologies, such as topic analysis, citation chasing, evidence extraction, natural language processing and data visualization, selecting the best analytical tools for your project's objectives.
We use a transparent and traceable process, from discovery through extraction and summarization to contextual analysis, to transform complex information into actionable insights.
Developing

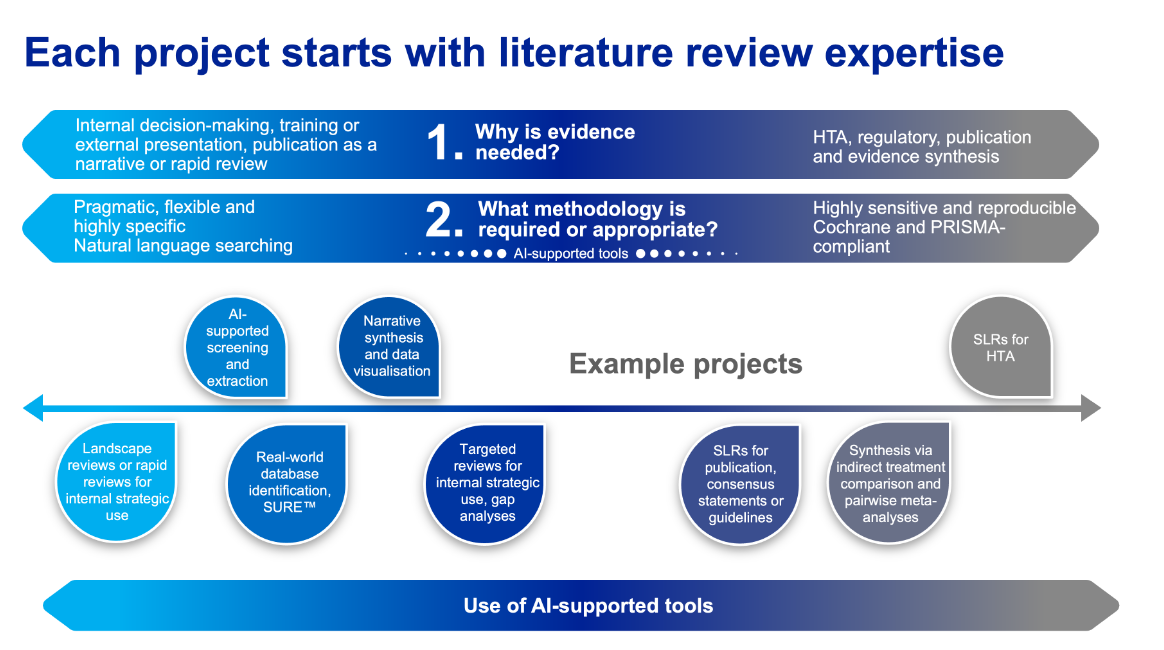
Literature reviews with AI assistance
Our systematic literature review team advises on the optimal approach to evidence review and synthesis, recommending appropriate methodology based on the evidence need, based on our deep understanding of systematic literature review (SLR) methods, techniques, good practice guidance and the shifting landscape of AI acceptance.
Literature reviews with AI assistance
We support literature reviews at scale using novel, robust AI approaches that leverage our domain expertise to process large volumes of information while maintaining efficiency and quality, depending on evidence needs and taking direction from industry guidelines.
Our reviews integrate into ITCs, HTAs, value dossiers or publications.
Evidence synthesis
Our technical experts and trusted partners advise on the optimal approach for meta-analysis and indirect treatment comparison, developing HTA-compliant feasibility analyses, indirect treatment comparisons and meta-analyses.
Evidence synthesis
Copyright license: www.creativecommons.org/licenses/by/4.0
Kittai, Adam S.; Skarbnik, Alan; Miranda, Miguel; Yong, Alan; Roos, Jack; Hettle, Robert; et al. (2023). A matching-adjusted indirect comparison of acalabrutinib versus zanubrutinib in relapsed or refractory chronic lymphocytic leukemia. figshare. Media. www.doi.org/10.6084/m9.figshare.24250873.v2

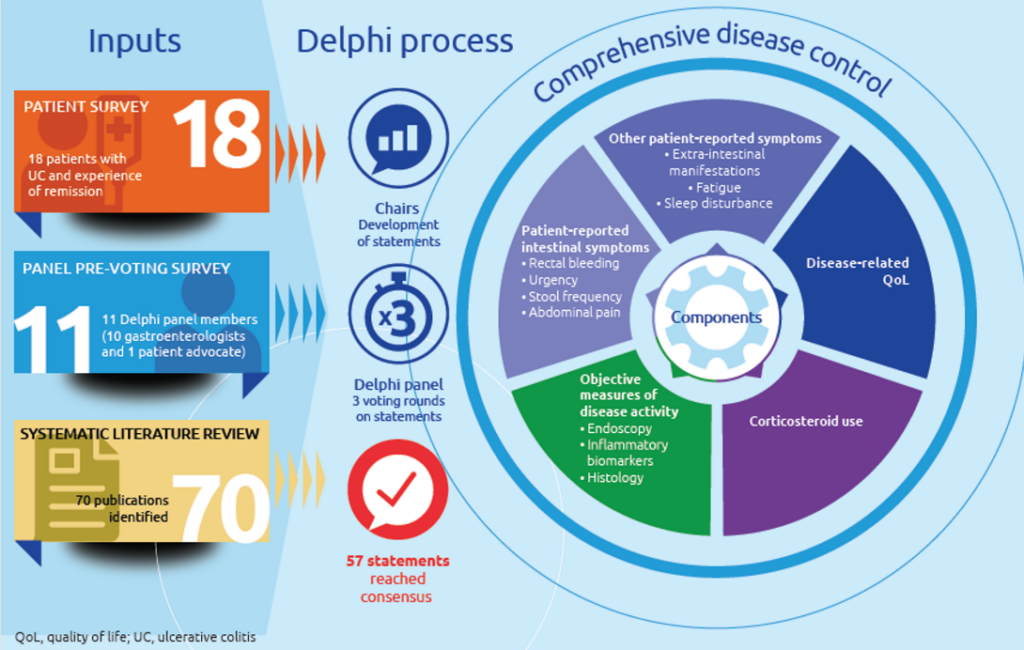
Consensus statements
We have a strong history of delivering consensus initiatives, including Delphi consensus statements involving patients and HCPs, that influence decision-making.
We are a leading partner in the ACCORD study to establish a reporting guideline for consensus initiatives. Find out more here.
Consensus statements
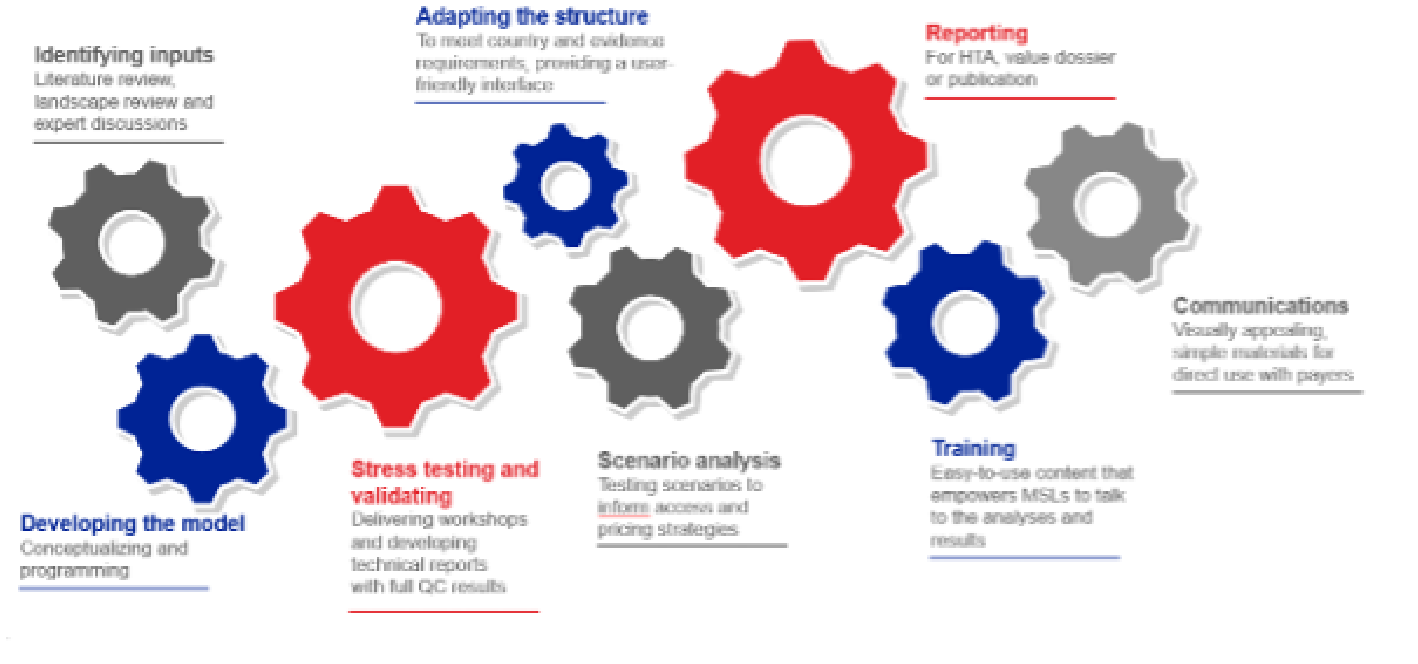
Modelling
Our technical experts and trusted partners support economic model development and adaptation, from identifying inputs, developing, adapting, stress-testing and scenario analysis.
Our economic model support – gearing up from analysis to communication
-
Identifying inputs
Literature review, landscape review and expert discussions -
Adapting the structure
To meet country and evidence requirements, providing a user-friendly interface -
Reporting
For HTA, value dossier or publication -
Developing the model
Conceptualizing and programming -
Stress testing and validating
Delivering workshops and developing technical reports with full QC results -
Scenario analysis
Testing scenarios to inform access and pricing strategies -
Training
Easy-to-use content that empowers MSLs to talk to the analyses and results -
Communications
Visually appealing, simple materials for direct use with payers
Communicating

Payer communications
We integrate evidence with strategy to create a compelling value story with the precision that payers need.
Based on deep technical expertise, we communicate complex analyses to non-statistical audiences, so they understand the real-life implications of product uptake on patients and on healthcare systems.
Payer communications
We develop compelling communications, including value dossiers, value story decks and objection handlers to support teams in communicating the value of your product. We create payer-facing tools that are evidence-based, visually engaging and easy to use, including interactive economic models and animations of complex ITC methodology.

HTA and JCA
We support HTA from start to finish, from access strategy, advisory workshops, submission drafting, modelling, preparation for negotiations and post-submission activities, working under tight timelines to develop compelling submissions.
HTA and JCA
We are ready for JCA and have a thought-out process for early population of the dossier and SLR, to manage workloads in the run-up to submission.
We have global coverage, for HTA, JCA and AMCP, understanding differences in payer evidence needs and creating efficiencies across submissions.
Alongside our patient engagement team, we make sure patient voices are heard in HTA, by developing summary information for patient groups and having contributed to HTAi guidance.

Publications
We have expertise in developing and reporting all the study types included in an HEOR publication plan, including SLRs, real-world studies, health economic models, patient-reported outcomes and indirect treatment comparison.
Publications
We enjoy working with authors to push for policy change, explaining where some requirements for payer evidence can be add significant delay to patient access.
We are experts in publication strategy and planning, especially integrating HEOR studies with the wider product strategy.
We go beyond the publication, widening the reach and impact of the research by developing animations, webinars and graphical abstracts.
Capability building
We design bespoke training, including self-directed and live learning programmes covering HEOR, RWE and market access to equip internal teams and functions with key knowledge and capabilities.
Capability building
Our training programmes for HEOR, RWE and market access are grounded in evidence-based learning design principles, ensuring that educational activities are both engaging and effective. From the outset, we involve learners to identify their challenges and knowledge gaps, ensuring that the material resonates with the audience. We are also passionate about infusing creativity and storytelling into our learning programmes, making our e-learning and workshop content come alive. This approach aims to inspire and drive long-lasting behaviour change, providing an enriching and impactful learning experience.



Contact us
Want to know more about us?
Oxford PharmaGenesis is a HealthScience communications consultancy. We are the largest independently owned and financed company in the healthcare communications sector. Founded in 1998, our award-winning organization now comprises more than 500 talented people working from offices and homes worldwide.
